Definition:
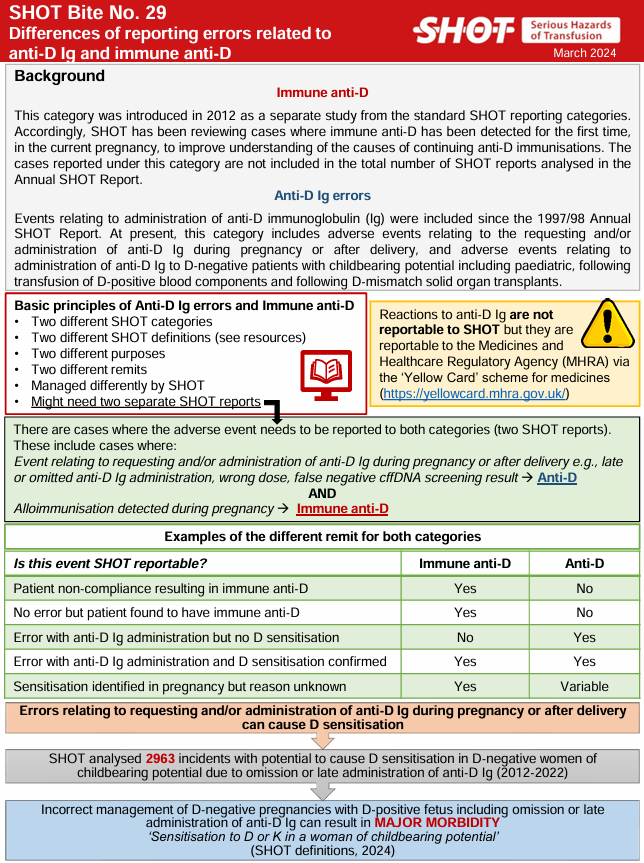
Anti-D immunoglobulin (Ig) errors refer to events relating to the requesting and/or administration of anti-D Ig and routine antenatal anti-D Ig prophylaxis (RAADP) during pregnancy and after delivery. It also includes events relating to the administration of anti-D Ig following inadvertent transfusion of D-mismatched red cells or platelets, as per national guidance.
For further details and examples of what to report, please visit the SHOT Definitions page.
Anti-D Immunoglobulin (Ig) Events in SHOT Reporting
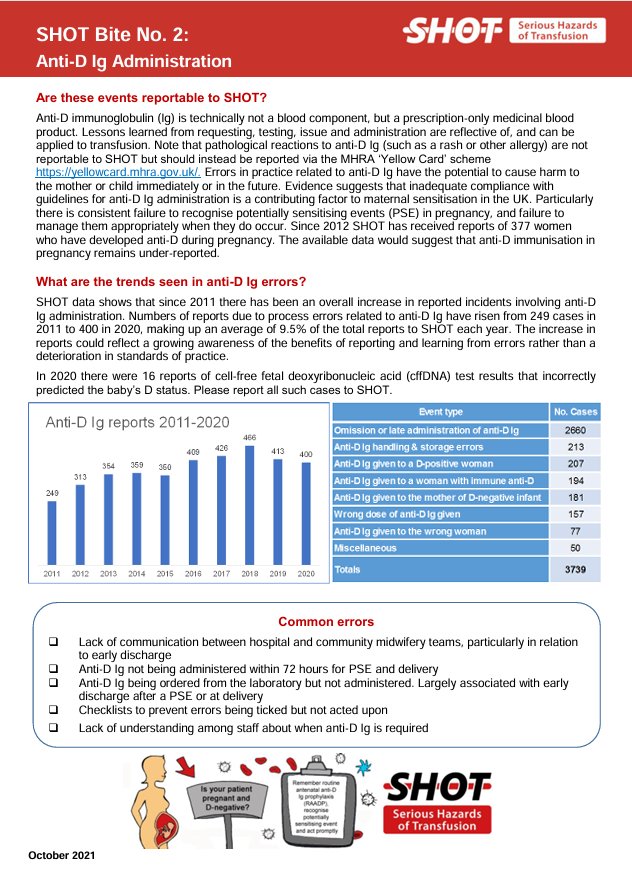
Events relating to the administration of anti-D immunoglobulin (Ig) have been included in the Annual SHOT Report since 1997/98. Initially, these events were reported under the Incorrect Blood Component Transfused (IBCT) category. In 2007, due to their distinct nature and significance, they were assigned a dedicated chapter.
Currently, this category encompasses adverse events associated with:
- All the steps of the transfusion pathway from requesting/prescription to administration of anti-D Ig during pregnancy or following delivery
- The administration of anti-D Ig to D-negative patients with childbearing potential, including paediatric patients:
- After transfusion of D-positive blood components
- Following D-mismatched solid organ transplant
These events are monitored due to the risk of alloimmunisation (i.e. anti-D development), which can have serious implications for future pregnancies and transfusion compatibility. For further information about cases where alloimmunisation has occurred, please see the chapter on ‘Immune Anti-D in Pregnancy’ in each years Annual SHOT Report.
SHOT Transfusion Safety Standards
The SHOT Transfusion Safety Standards were released in July 2025, and these replace recommendations in the Annual SHOT Reports. More details on these can be found at: https://www.shotuk.org/transfusion-safety/transfusion-safety-standards/
Recommendations from recent Annual SHOT Reports (prior to 2024)
- Interoperability between LIMS, including reference laboratory, and maternity systems reduces risk of transcription errors and should be implemented
- Organisations should review current processes to identify gaps where improvements could be implemented to support safe practice
- Processes should be in place that support recognition of the need for anti-D Ig in non-gynaecology and maternity settings
Action: Laboratory management, IT departments, maternity services, reference laboratories
Please see the individual SHOT chapters at the end of the page for other previous recommendations which remain relevant.
Anti-D Ig errors SHOT resources
Guidelines relating to anti-D Ig
SHOT is aware that the current guidelines relating to anti-D Ig are under review. The relevant SHOT resources will be reviewed and updated in due course following the publication of the new guidelines.
Anti-D Immunoglobulin errors and immunisation in pregnancy: Insights from SHOT
Meet the experts webinar– Anti-D immunoglobulin (Ig) errors and immune anti-D (September 2025)

Immunoglobulin during the perinatal period (including GAP analysis tool)



Email signatures: Messages related to Anti-D
Cell-free fetal DNA (cffDNA) resources
Cell free fetal DNA (cffDNA) discrepancy investigation form
SHOT Bite No.28: Cell-free fetal DNA (cffDNA) screening errors
IT resources
How information technology systems can support safe practice in anti-D Ig management in pregnancy
External resource (cffDNA): Pilot electronic request for NHSBT reference laboratory tests
External resources
Qureshi, H., Massey, E., Kirwan, D., Davies, T., Robson, S., White, J., Jones, J. and Allard, S. (2014), BCSH guideline for the use of anti-D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfusion Med, 24: 8-20. BCSH guideline for the use of anti‐D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn – Qureshi – 2014 – Transfusion Medicine – Wiley Online Library
Austin, E. et al., 2009. Guidelines for the Estimation of Fetomaternal Haemorrhage. British Committee for Standards in Haematology (BCSH), pp. 1-23. Available at: The Estimation of Fetomaternal Haemorrhage
NICE TA156 – Routine antenatal anti-D prophylaxis for women who are rhesus D negative
NICE DG25 – High-throughput non-invasive prenatal testing for fetal RHD genotype
NICE NG126 – Section 1.7 Anti-D immunoglobulin prophylaxis
NICE NG140 – Section 1.3 Anti-D prophylaxis
NICE NG126 – Ectopic pregnancy and miscarriage: diagnosis and initial management
Maternity Services Monthly Statistics – NHS England Digital
Patient information leaflets – to help staff with the informed decision-making/consent process
The anti-D national comparative audit is scheduled for April 2026 (National Comparative Audit – Hospitals and Science – NHSBT) and the report will be added to this page on due course.
Anti-D Annual Report Chapters
To access the chapter click either on the cover of the Annual SHOT Report or the link below the picture.