Definition:
Avoidable Transfusion
Where the intended transfusion is carried out, and the blood component itself is suitable for transfusion and compatible with the patient, but where the decision leading to the transfusion is flawed. Every unit transfused should be an individual decision, so this might include transfusion of multiple units where not all were appropriate/necessary.
Delayed Transfusion
Where a transfusion of a blood component was clinically indicated but was not undertaken or non-availability of blood components led to a significant delay (e.g., that caused patient harm, resulted in admission to ward or return on another occasion for transfusion).
Under or Overtransfusion
A dose inappropriate for the patient’s needs, excluding those cases which result in transfusion-associated circulatory overload (TACO)and usually resulting in a haemoglobin or platelet level significantly outside the intended target range. Infusion pump errors leading to under or over transfusion with clinical consequences (if no clinical consequences please report as handling and storage errors (HSE)).
Prothrombin Complex Concentrates (PCC)
Reporters are asked to report any issues with the prescription and administration of prothrombin complex concentrate. This includes delays in administration, inappropriate prescription or problems with administration. (Excludes allergic reactions which should be reported under the yellow card scheme to the MHRA).
SHOT first saw reports of ‘inappropriate or unnecessary’ transfusions in 1999/00, which were at that time included in the Incorrect Blood Component Transfused (IBCT) category. From 2003 the cases were described in a separate sub-section within the IBCT chapter, and from 2008 had a dedicated chapter in the Annual SHOT Report ‘Inappropriate and Unnecessary Transfusion (I & U)’. SHOT began receiving reports of delayed transfusions in 2010 after the recommendation from the NPSA Rapid Response Report, and in 2012, the category was re-named ‘Avoidable, Delayed or Undertransfusion (ADU) to better reflect the increasing number of reports of delayed transfusions being received. Incidents related to prothrombin complex concentrates (PCC) were first seen in 2014.
Recent Recommendations
Clear guidelines for patients being transferred between hospital departments, or between hospitals must be available and followed to ensure patient safety. This should include the need for adequately trained and skilled staff to supervise the transfer.
Major haemorrhage protocols should be reviewed and practiced end-to-end with drills to ensure that they are workable, and that staff are familiar with them
Action: Hospital chief executive officers, transfusion laboratory managers, hospital transfusion committees
Activation of MHP should be simple and standardised to avoid issues with hospital-specific procedures
Hospitals should review their MHP and test them with drills and simulation to ensure they are fit for purpose. This should cover all the steps in the process from end-to-end and must include all staff groups involved
MHP activations should be followed by a debrief with everyone involved to identify what went well and what could be improved
Transfusion professionals should work closely with higher education institutes to ensure that the courses they are offering are fit for purpose and ensure all staff are equipped with the skills and knowledge they require to deliver safe transfusions
Action: Hospital transfusion committees, higher education institutes
Training in major haemorrhage protocols should be multidisciplinary and include all staff involved when MHP is activated
Training should emphasise that group O red cells are only used when group-specific or crossmatched red cells are not readily available
Paediatric transfusion protocols should be readily accessible to all clinical staff
Hospitals should have clear guidelines for patients being transferred between hospitals to reduce the risk of adverse outcomes
Action: Hospital transfusion teams
The ED should ensure they have a protocol with clear instructions for dose and administration of PCC. Staff should be appropriately trained in their use
A standardised single first dose for emergency use should be adopted to reduce PCC administration delays in urgent situations
Use of PCC should be regularly audited for timeliness and appropriateness
Action: Medical directors, hospital transfusion teams, audit leads
ADU SHOT Resources
ADU – Under or overtransfusion Case Studies
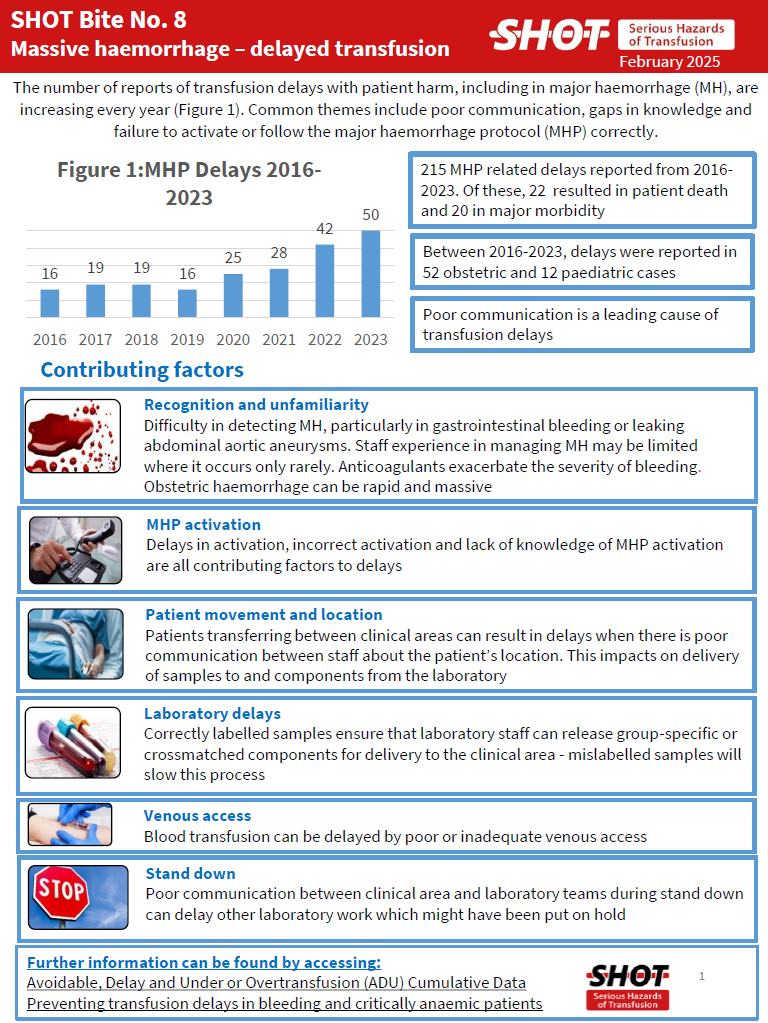
SHOT Bite No. 8: Massive Haemorrhage – Delays
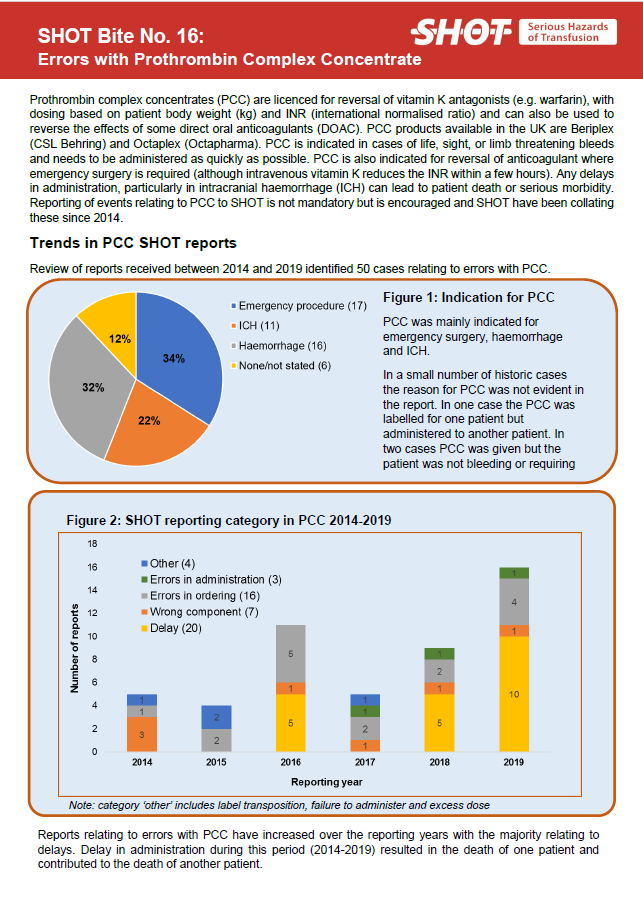
SHOT Bite No. 16: Errors with Prothrombin Complex Concentrate
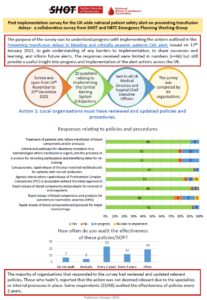
Relevant Resources
Haematological management of major haemorrhage: a British Society for Haematology Guideline
Acute care toolkit 1: Handover
ADU Annual Report Chapters
To access the chapter click either on the cover of the Annual SHOT Report or the link below the picture





















Avoidable, Delayed or Under/Overtransfusion (ADU) 2023
Avoidable, Delayed or Under/Overtransfusion (ADU) 2022
Avoidable, Delayed or Under/Overtransfusion (ADU) 2021
Avoidable, Delayed or Under/Overtransfusion (ADU) 2020
Avoidable, Delayed or Under/Overtransfusion (ADU) 2019
Avoidable, Delayed or Under/Overtransfusion (ADU) 2018
Avoidable, Delayed or Under/Overtransfusion (ADU) 2017
Avoidable, Delayed or Under/Overtransfusion (ADU) 2016
Avoidable, Delayed or Under/Overtransfusion (ADU) 2015
Avoidable, Delayed or Under/Overtransfusion (ADU) 2014
Avoidable, Delayed or Under/Overtransfusion (ADU) 2013
Avoidable, Delayed or Under/Overtransfusion (ADU) 2012
Inappropriate Unnecessary or Under Delayed Transfusion (I&U) 2011
Inappropriate Unnecessary or Under Delayed Transfusion (I&U) 2010
Inappropriate Unnecessary or Under Delayed Transfusion (I&U) 2009
Inappropriate and Unnecessary Transfusion (I&U) 2008
Inappropriate and Unnecessary Transfusion (I&U) 2007
Inappropriate and Unnecessary Transfusion (I&U) 2006
Inappropriate and Unnecessary Transfusion (I&U) 2005